Ribosomally synthesized and post-translationally modified peptides (RiPPs) are a rapidly growing group of natural product that vary in length from ~20 to 110 amino acids. Precursor peptide formed from ribosomal synthesis is cleaved by peptidase into a leader and core peptide. Extensive post-translational modifications (PTMs) of the core peptide results in structurally and chemically diverse groups possessing a wide range of bioactivities. Common PTMs of the core peptide are dehydration, prenylation, acetylation, heterocyclization and macrocyclization. These small peptides have many therapeutical and other industrial applications. A classical example is Nisin (a lanthipeptide), that is used in food industry as food preservative. Some of other antibiotics include plantazolicin, cytotoxin and bottromycin.
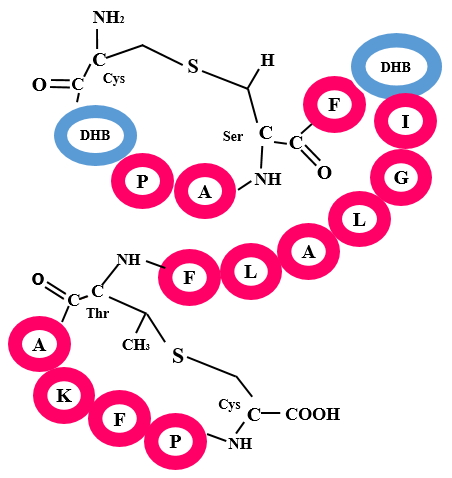
Lanthipeptides are one of the major classes of RiPPs containing characteristic lanthionine (Lan) and methyl-lanthionine (MeLan) moieties. Lan and MeLan are formed after dehydration of Serine and Threonine residues respectively and subsequent cyclization with Cysteine residues. Such cross-links are formed by a Ser/Thr-Cys pair via a thioether linkage that connects their β-carbons. Lanthipeptides are further divided into four categories i.e. lanthipeptide A, lanthipeptide B, lanthipeptide C and lanthipeptide D on the basis of enzymes involved in the formation of crosslinking. In lanthipeptide A, two separate enzymes are involved in dehydration (LanB) and cyclization (LanC) steps whereas, in other classes a single multifunctional enzyme is involved for both the reactions.
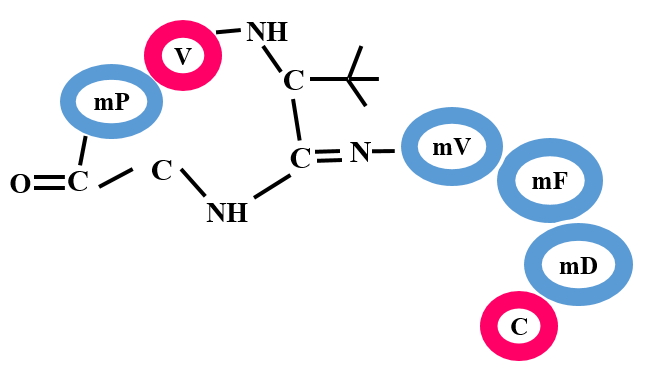
Bottromycins are known for their antimicrobial activity. They contain structural features like macrocyclic amidine and a decarboxylated C-terminal thiazole. The biosynthesis of bottromycin is quite different as compared to other RiPP classes. Precursor peptides belonging to this class contain a C-terminal follower peptide (35-37) instead of N-terminal leader peptide. Bottromycins are effective against methicillin resistant S. aureus (MRSA) and vancomycin-resistant Enterococci (VRE) and other gram-positive bacteria.
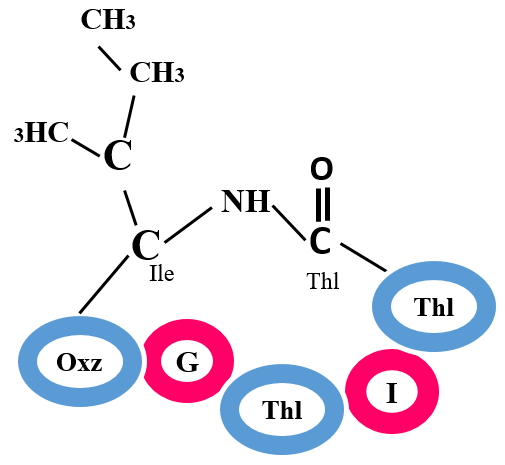
Cyanobactins are small bioactive peptides which are derived from cyanobacteria or other marine animals. The core peptides are macrocyclized from N-to-C terminal. Depending on a signature motif (RSI) in leader peptide, Cysteine(s) and Serine(s) present in core region, are heterocyclized to form thiazol(in)e and oxazol(in)e residues. Modifications in cyanobactins are also seen in terms of prenylation on Serine, Threonine and Tyrosine and N-methylation of Histidine.
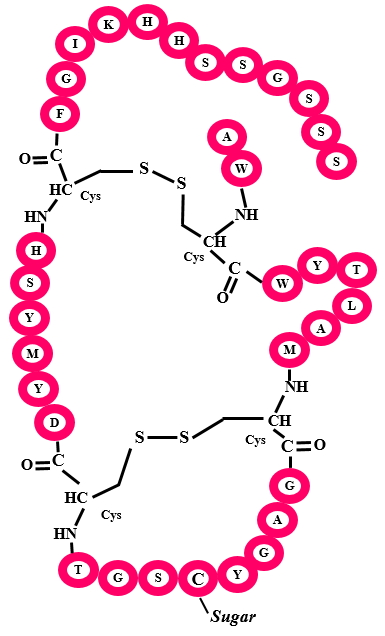
Glycocins are antimicrobial peptides where a glucose group is transferred onto a Cysteine residue by glcosyltransferases. Other Cysteine residues are involved in disulfide linkages. To date only two glycocins have been characterized namely Glycocin F and Sublancin 168.
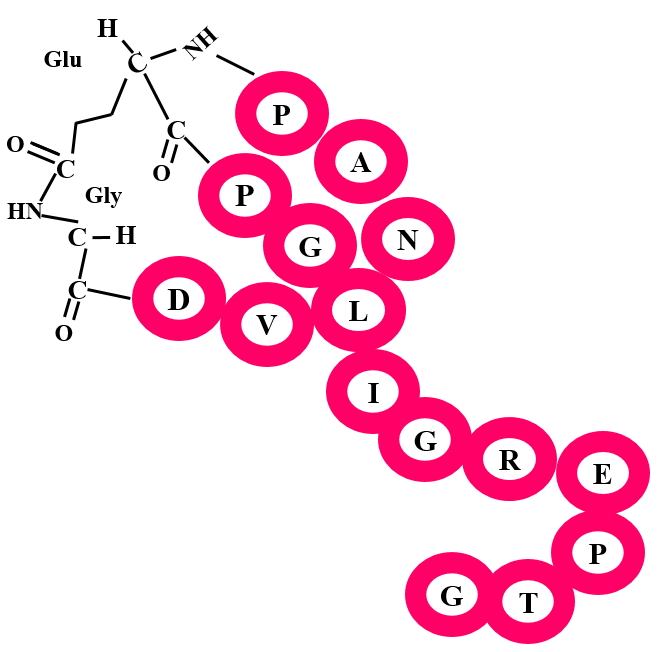
Lassopeptides are defined by the presence of a knotted structure known as “lasso fold”. They usually vary in length from 16-21 residues. The cross-links (marcolactamization) in lassopeptides are formed by the condensation of the N-terminal amino group with a side-chain carbon of a Glu or Asp residues at 8 or 9 position. The lactam formation leads to the irreversible knotted structure.
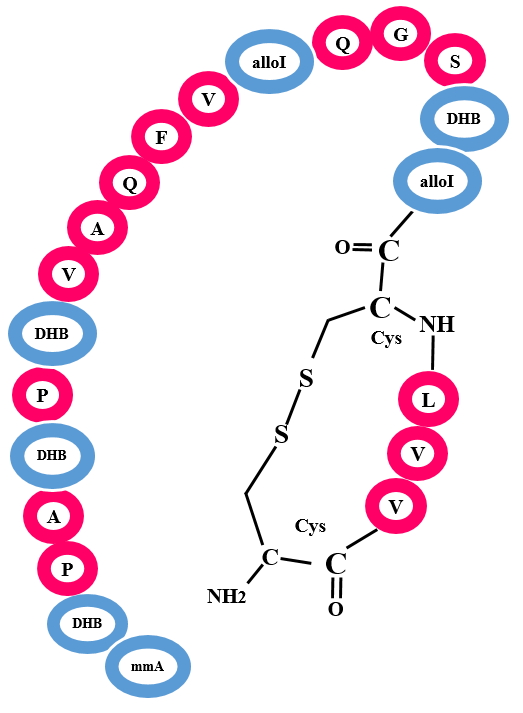
Linaridins are defined by the presence of thioether cross-links similar to lanthipeptides, but this class differ in its biosynthetic machinery. Presence of any lanthipeptide-like dehydratases are not found in Linaridins gene cluster. Some of the characterized Linaridins are Cypemycin, Grisemycin, Linaridin, Htur_3018 etc.
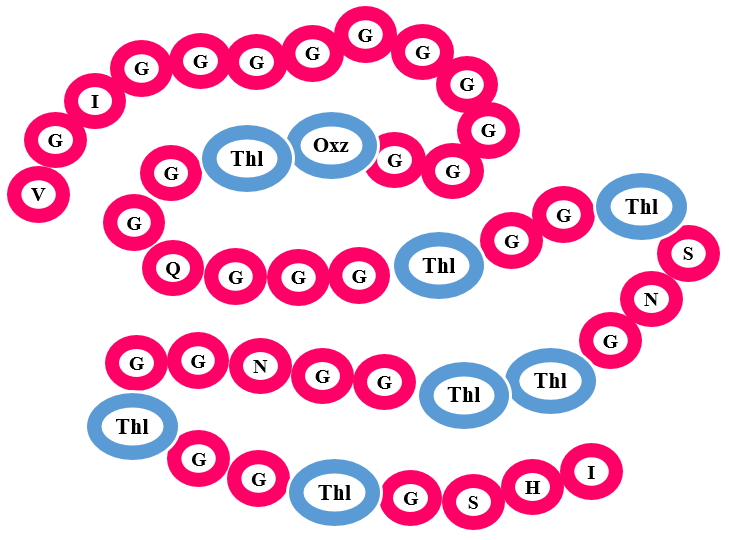
Linearazols have several Thiazol(in)e and (methyl)-Oxazol(in)e in their structure. These hetrocycles are derived from Cysteine, Threonine and Serine residues. Apart from Azol(in)e rings other modifications can also occur such as acetylation, methylation and dehydration
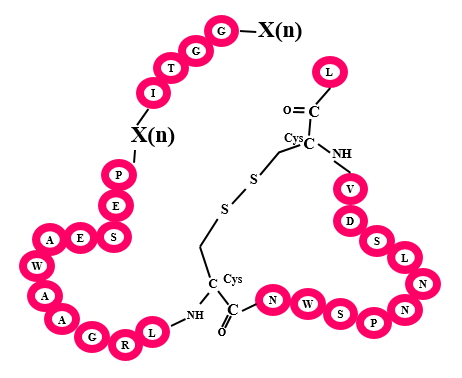
Microcins are mostly plasmid encoded antimicrobial peptides from Enterobacteriaceae family. There are two classes of Microcins, class I and class II which are distinguished based on their molecular masses. Class I peptide have Molecular mass of less than 5KDa while Class II peptides' molecular mass range between 5-10kDa.
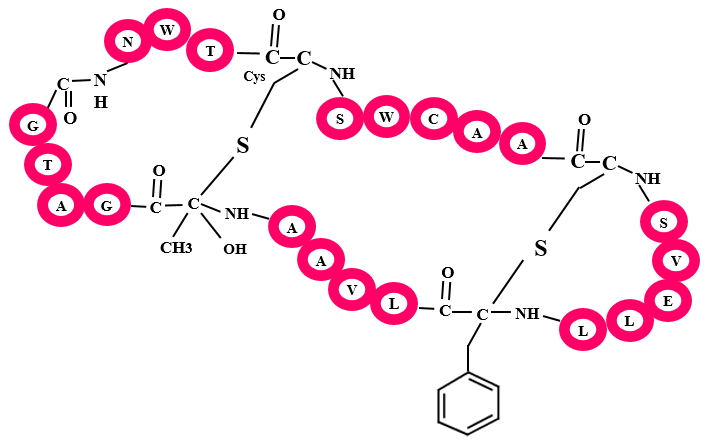
In Sactipeptides, Cysteine side chain is crosslinked with α-carbon of any residue. Subtilosin A, thurincin H, are few examples of this class. Sactipeptides are commanly known as "Sulfur to α-carbon antibiotics".
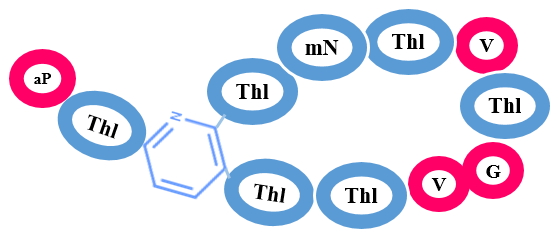
Thiopeptides are characterized by the presence of several Thiazol(in)e rings along with Nitrogenous ring that can be found in three forms - a piperidine, dehydropiperidine and pyridine. Thiopeptides shows anti-bacterial activity against gram-positive bacteria along with anticancer and immunosuppresive activity. Few effective thiopeptide antibiotics are Sch 18640, thiostrepton, Sch 40832, GE2270 A, nosiheptide etc.
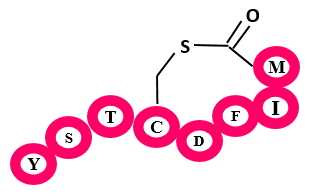
Auto Inducing Peptides (AIPs) are mainly produced by Firmicutes and they have thioester as a conserved feature in their structure which distinguish them from other class. These are quorum sensing compounds. The precursor peptide of AIPs has three differnt regions an N-terminal amphiphatic helical region, a core peptide that encodes the residues that will form the mature AIP, and a C-terminal, highly negatively charged region.
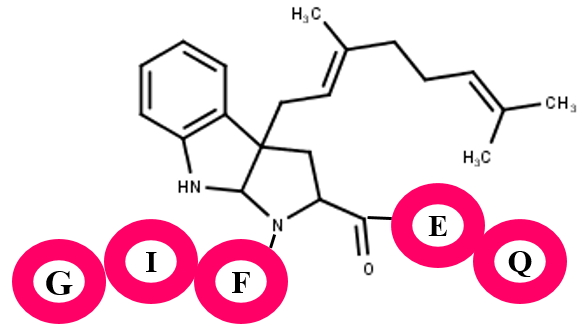
This is another quorum sensing RiPP. The biosynthesis of these compounds take place from much larger presursor peptides and has a highly conserved tryptophan residue.
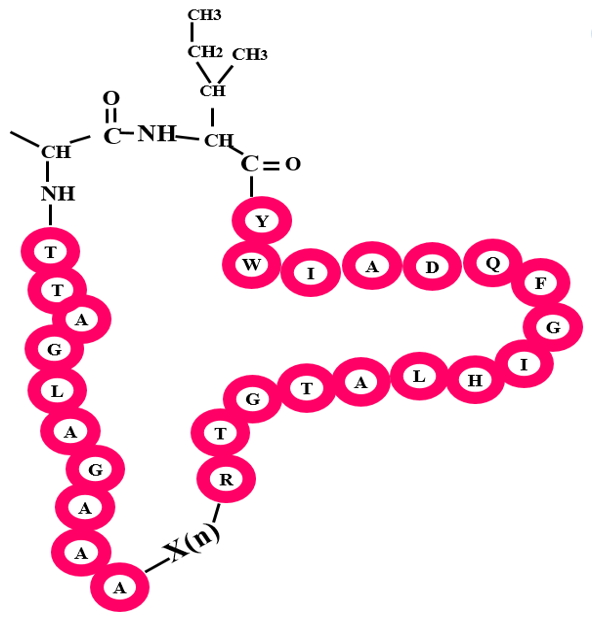
Bacterial head-to-tail peptides deal with comparatively larger peptides (35-70 residues) which cyclizes from C to N terminal. They differ from other classes such as Cyanobactins in their size and biosynthetic pathway that leads to macrocyclization. Few bacterial head to tail peptides include butyrivibriocin AR10, Acidocin B, Amylocyclicin etc.